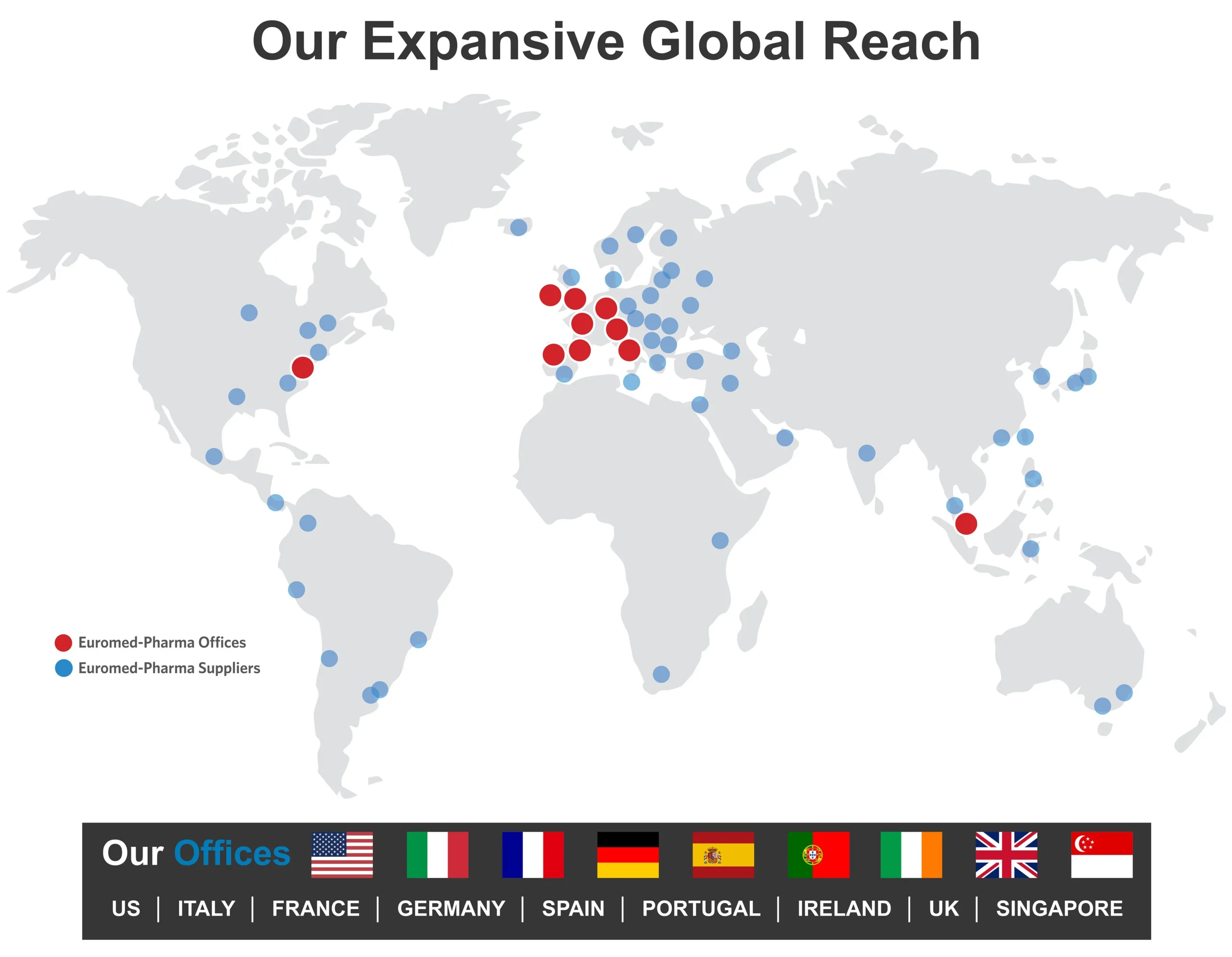
Our procurement network ensures excellent global and best-in-class European access to pharmaceutical products for clinical trials
Contact UsOur procurement network ensures excellent global and best-in-class European access to pharmaceutical products for clinical trials
Contact UsExplore Our Expansive Global Procurement Network
Clinical Trial Services at Euromed Pharma US
Euromed Pharma US offers a wide range of services to support you throughout the lifecycle of your study:
- Our procurement team of 25+ individuals allows us excellent access globally to the drugs and materials you need for your clinical studies.
- We offer primary and secondary packing and labeling services to maintain regulatory compliance of your clinical trial products.
- Our labeling capabilities allow us to label at -80° C.
- We guarantee that your clinical trial supplies are stored under specific conditions and delivered promptly to their destinations.
- Our storage capabilities allow us to store at -80° C.
- As your Importer of Record, we manage all import-related activities, including customs clearance, ensuring that your clinical trial supplies are delivered without delay.
- We handle the return process from clinical sites, oversee the reception and reconciliation of returned materials, and coordinate their destruction through locally approved and qualified vendors.
- We ensure all necessary documentation, including destruction certificates, is provided to maintain traceability of the destroyed clinical supplies.
- Our high-quality placebo manufacturing services are designed to meet your specific trial requirements.
- We offer placebo manufacturing as well as over-encapsulation depending on your specific trial requirements.
Clinical Trial Drug Sourcing Capabilities
With 9 offices located throughout the world, we offer you excellent access to clinical trial supplies globally and unparalleled access to supplies in Europe.
Since drug prices in Europe are significantly lower than in the US, we help you achieve substantial cost savings all while remaining fully compliant with FDA regulations, which permit the importation of clinical trial supplies for use in investigational studies.
Partner with us to enhance your clinical trial efficiency and reduce expenses.
Connect With Us In Person



Clinical Trial Services in the US
At Euromed Pharma US, we provide end-to-end support for your clinical trials. Our services include strategic consulting on drug procurement, comparator and ancillary sourcing, and the management of Investigational Medicinal Products (IMPs). We offer clinical manufacturing, packaging, and labeling services, ensuring compliance with regulatory standards.
Our robust logistics network guarantees timely delivery and continuous supply chain management, minimizing the risk of delays. Trust us as your reliable partner in every phase of your clinical research.
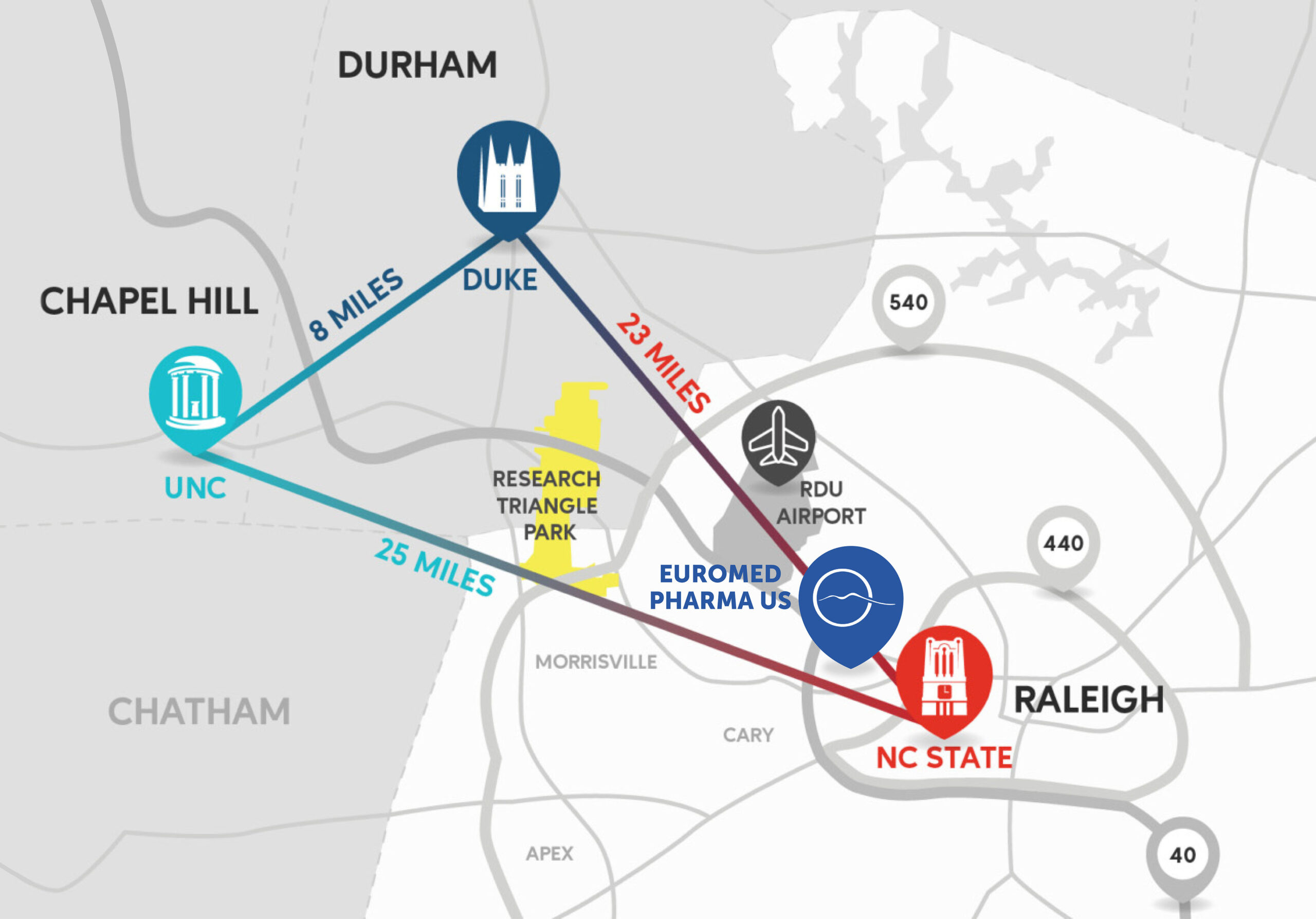
Euromed Pharma US Location
Euromed Pharma US is conveniently located close to Research Triangle Park (RTP) and the RDU airport.
Want to Learn More?
Connect with a Euromed Pharma US Team Member
Privacy Policy – Cookie Policy – Whistleblowing – Euromed Pharma a Petrone Group Company®